2.1.
Allocation of the symmetry
planes
2.1.1.
The properties of the peptide bond
Double bond in the peptide groups, according to [15] can be in two positions:
O=C–N– < ---
> –O–C=N+
According to L. Pauling (cit. in [15]), this effect
is caused by resonance of two limiting structures.
Due to the resonance peptide group in proteins is flat [15] .From the standpoint of quantum
mechanics, the electron clouds in such resonance structures have the form of
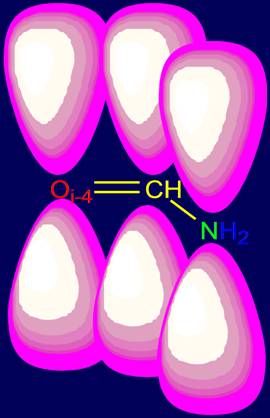
overlapping dumbbells (Fig. 2) – of sp2-hybridized clouds.
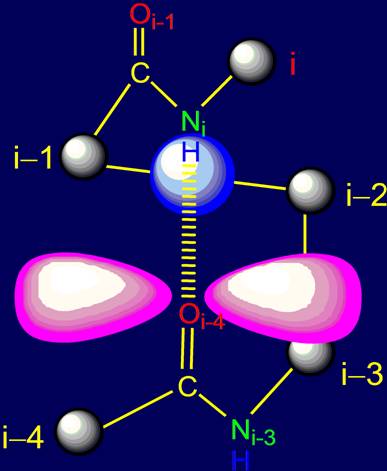
The only hydrogen bond, leading to a
cyclic pentafragment, formed between atoms NiH….Oi-4=C of the two peptide
groups of the protein polypeptide chain. In the hydrogen bonding of these
groups involved a hydrogen atom, which has a spherical S-orbital associated
with the nitrogen atom of the Ni i-th alpha-carbon atom and
an oxygen atom Oi-4 of peptide bond of i-4-th alpha-carbon atom. View of electron
clouds in this region is shown in Figure 3. It is this area is the subject of
our analysis.
|
|
|
|
Fig. 2. The shape of the electron clouds of the peptide bond. |
Fig.
3. The shape of
the electron clouds in the hydrogen bond
NiH….Oi-4=C of
the two peptide groups
of pentafragment (top view). The gray balls
– alpha-carbon atoms. |
2.1.2.
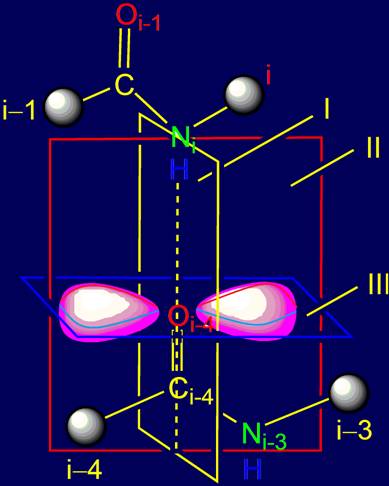
Carrying out the planes of symmetry in the area of bond NiH….Oi-4=C
Through the bond NiH….Oi-4=C three mutually
perpendicular planes can be drawn (Fig. 4). The plane I (marked in yellow) goes
through this bond perpendicularly to the plane
of sheet;
as a result each of the sp2-hybridized cloud blades will be
symmetrical on both sides of the atom Oi-4. Plane II (marked in
red) runs parallel to the plane of the sheet (perpendicular to the plane I) and
separates the sp2-hybridized cloud into two symmetrical halves - the
front and back. Plane III (marked in blue) is perpendicular to the previous two
planes and separates each blade of the sp2-hybridized cloud into two
halves - top and bottom.
|
|
|
Fig.
4. Allocation of the planes of symmetry in the area of bond NiH….Oi-4=C (top
view). |
In Section 2.2 it will be shown how with use of the allocated planes of symmetry 20
vectors of action of physical operators can be received.
Address for connection: vector-machine@narod.ru